Dear all,
The FSHD ETN hopes that you have made a good start of 2024!! We are very pleased with the steps already taken and work that has been done, here we give you a short update on our activities and plans for this year.
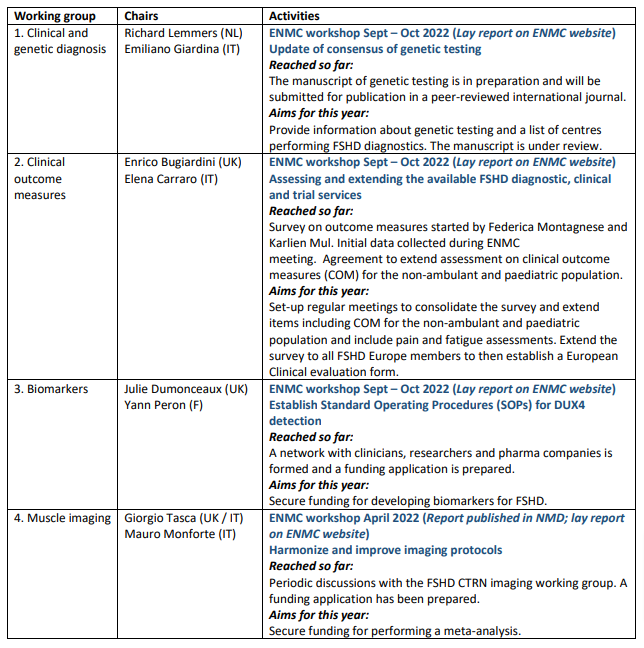
Update of the working groups activities:
Other news:
Project Mercury – The Global Initiative to Speed the Delivery of Therapies for FSHD

FSHD Europe and the ETN are very pleased to be part of Project Mercury, a collaboration program led by FSHD patient advocacy organizations in partnership with experts, biopharma companies and researchers from all over the world. This project is focused on solving specific challengesthat slow or stop the development and delivery of therapies to people affected by FSHD around the world. The Mercury project is led by a global task force, in collaboration with working groups in different countries and regions. In Europe the following countries are included: United Kingdom (UK), France, Spain, Italy, Germany, and The Netherlands. Other countries are Canada, United States, Brazil and Australia. See for more information: (Project Mercury – The Global Initiative to Speed the Delivery of Therapies for FSHD (projectmercuryfshd.org)). Project Mercury is part of the Patient Lifestyle and Disease Data Interactium (PaLaDIn) project. PaLaDIn is funded by the Innovative Health Initiative (IHI) with contributions from TREAT-NMD, the FSHD Society and UK Research and Innovation and aims to accelerate research, understanding and treatment for neuromuscular and other rare diseases. See for more information: TREAT-NMD and collaborators launch a €21 million Patient Lifestyle and Disease Data Interactium (PaLaDIn) Project. – TREAT-NMD
FSHD Europe and the ETN are very pleased to be part of Project Mercury, a collaboration program led by FSHD patient advocacy organizations in partnership with experts, biopharma companies and researchers from all over the world. This project is focused on solving specific challengesthat slow or stop the development and delivery of therapies to people affected by FSHD around the world. The Mercury project is led by a global task force, in collaboration with working groups in different countries and regions. In Europe the following countries are included: United Kingdom (UK), France, Spain, Italy, Germany, and The Netherlands. Other countries are Canada, United States, Brazil and Australia. See for more information: (Project Mercury – The Global Initiative to Speed the Delivery of Therapies for FSHD (projectmercuryfshd.org)). Project Mercury is part of the Patient Lifestyle and Disease Data Interactium (PaLaDIn) project. PaLaDIn is funded by the Innovative Health Initiative (IHI) with contributions from TREAT-NMD, the FSHD Society and UK Research and Innovation and aims to accelerate research, understanding and treatment for neuromuscular and other rare diseases. See for more information: TREAT-NMD and collaborators launch a €21 million Patient Lifestyle and Disease Data Interactium (PaLaDIn) Project. – TREAT-NMD
The FSHD patient survey is published in the journal Neuromuscular Disorders.
The article can be found here: Facioscapulohumeral Muscular Dystrophy European Patient Survey: Assessing Patient Reported Disease Burden and Preferences in Clinical Trial Participation – PubMed (nih.gov)
A welcome to our new project manager.
We are pleased to announce the appointment of Ria de Haas as project manager for both FSHD Europe
and the FSHD ETN. Ria will be supporting the development of the ETN and FSHD Europe to:
- Support more clinical trials for FSHD in Europe
- Develop the membership of both FSHD Europe and the ETN
- Promote closer collaboration of all stakeholders to deliver a treatment for FSHD Ria previously worked at the Dutch patient organization Spierziekten Nederland and is now based at the Radboud University Medical Centre working with Nicol Voermans (chair ETN) and Sheila Hawkins (chair FSHD Europe).
She can be contacted at: ria@fshd-europe.info
Preparation of an update of the international standard of care.
FSHD ETN together with FSHD society and EURO-NMD is preparing an update of the international standard of care. By using a standard evidence-based approach, based on the Dutch FSHD guideline, the goal is to help neurologists and other physicians to understand how to confirm the diagnosis of FSHD and guide standardised patient care and treatment plans, which are needed since there are many trials expected to roll out in the upcoming years. The guideline is expected to be completed this year. More information can be found here: Extraordinary Measures – Updating the standard of care for FSHD | FSHD Society
31st Annual FSHD Society International Research Congress, Denver, Colorado, June 13 – 14, 2024
World renowned clinicians, medical researchers, pharmaceutical industry leaders and basic scientists present and discuss new developments, reinforce collaborative efforts, facilitate new initiatives, and coordinate research and clinical activities. With the recent advances in FSHD research and clinical advances, this conference has become catalytic in translating ideas into potential therapies. The conference will be held in Denver, Colorado, June 13-14. More information can be found here: 31st Annual International Research Congress on FSHD | FSHD Society
Clinical trials in Europe
| Trial name | Drug | Company | Phase | Status | Trial sites in Europe |
| REACH | Losmapimod | Fulcrum | Phase 3 | Active, not recruiting | Denmark, France, Germany, Italy, Netherlands, Spain, UK |
| MANOUVRE | RO7204239 | Roche | Phase 2 | Recruiting | Denmark, Italy, UK |
| FORTITUDE | AOC 1020 | Avidity | Phase 1/ 2 | Recruiting | UK |
FSHD Europe welcomes two new member organisations!
Recently two national patient organizations, Muskelsvindfonden from Denmark and Spierziekten Vlaanderen from Belgium joined FSHD Europe as new member organizations. FSHD Europe is very pleased having now 9 members from 8 different countries (France, UK, Germany, Netherlands, Italy, Spain, Denmark, and Belgium). The aim is to include more European countries. If you know national patient organizations or patient groups involved in FSHD, not yet connected to FSHD Europe.
Please refer them to Ria de Haas (ria@fshd-europe.info).
FSHD University webinar featuring FSHD ETN chair Prof. Nicol Voermans
While FSHD patients wait for new drugs to slow the progression of muscle weakness, Nicol emphasizes that there are many interventions already that can have a significant positive benefit. She encourages everyone with FSHD to do what they can to be as healthy and “trial fit” as possible. Nicol was joined by Nathaniël Rasing, MD, a Research Physician at Radboud University Medical Centre. His primary focus is on studying facial weakness, communication, participation, and quality of life in FSHD. See for more information: Treating FSHD – A broader view | FSHD Society
We aim to have open membership and aim to involve experts from all European countries. You might be contacted by clinicians or researchers in your country who are interested or know (future) FSHD experts in European countries not yet involved in the ETN. Please refer them to Ria de Haas (ria@fshd-europe.info).
Best wishes,
The FSHD ETN Executive committee:
Teresinha Evangelista, Pascal Laforêt, Alexander Mejat,
María Vriens – Muñoz Bravo, Valeria Sansone and Nicol Voermans and the chairs of the WG 1,2,3 and 4
For questions: nicol.voermans@radboudumc.nl or ria@fshd-europe.info